What is myasthenia gravis?
The term ‘myasthenia gravis’ (MG) comes from the Greek word ‘myasthenia’ meaning muscle weakness and the Latin word ‘gravis’ meaning severe. It is an autoimmune condition which causes problems with the transmission of signals from the nerves to the muscles. This results in weak muscles that get tired quickly and which improve after rest.
In the early stages, myasthenia gravis mostly affects the muscles that control eye movement, facial expression, chewing, and swallowing. As the condition progresses, neck and limb muscles may also be affected causing difficulty with holding the head up, walking upstairs and raising the arms. If untreated, breathing may be affected. Fortunately treatment – which may include medication and/or surgery – is usually successful in managing the symptoms of the condition.
In this factsheet:
• What are the different types of myasthenia?
• How is myasthenia gravis diagnosed?
• What are the symptoms of myasthenia gravis?
• What complications can occur?
• Is it risky for women with myasthenia gravis to have children?
• Who gets myasthenia gravis?
• What causes myasthenia gravis?
• How is myasthenia gravis treated?
• What is the prognosis?
• Is there anything I can do to stop my symptoms getting worse?
• What research is being done?
What are the different types of myasthenia?
Generalised myasthenia gravis
The most common type is often referred to as ‘generalised myasthenia gravis’ where muscles throughout the body are affected to varying degrees. This factsheet mostly deals with this type of myasthenia gravis. About 15 percent of people with myasthenia gravis have what is called ‘ocular myasthenia gravis’ because only the eye muscles are affected.
Lambert Eaton myasthenic syndrome
Lambert Eaton myasthenic syndrome is a very rare form of myasthenia gravis. Like the more common type of myasthenia gravis it is also an autoimmune condition. People with Lambert Eaton myasthenic syndrome are also weak and fatigue easily, but eye symptoms are less common and it is often associated with a particular type of lung cancer.
Congenital myasthenia or congenital myasthenic syndrome
There is also a very rare related condition called ‘congenital myasthenia’ or ‘congenital myasthenic syndrome’. Unlike myasthenia gravis, this is not an autoimmune condition; babies are born with this inherited genetic condition.
How is myasthenia gravis diagnosed?
If a person’s physical examination and medical history reveals a pattern of weakness that suggests myasthenia gravis, further investigations are done to confirm the diagnosis:
- A blood test to detect the presence of antibodies to the acetylcholine receptor (AChR) or MuSK. The majority of patients have antibodies to one of these proteins and this confirms the diagnosis of myasthenia gravis (see ‘What causes myasthenia gravis?’ below).
- Electromyography (EMG) uses electrodes to stimulate muscles and evaluate muscle function. Muscle contractions that become progressively weaker may indicate myasthenia gravis.
- The ‘Tensilon test’ is often used to diagnose MG. It involves an injection of a drug called Tensilon which temporarily improves muscle strength in people with myasthenia gravis.
- Chest x-ray, CT scan or MRI may be performed to examine the thymus gland, because abnormalities of the thymus are often associated with myasthenia gravis.
What are the symptoms of myasthenia gravis?
Myasthenia gravis is a very variable condition with a wide range of severity and different people may experience weakness in different muscle groups. Reaction to treatment can vary as well.
Initial symptoms of myasthenia gravis may include difficulty speaking (dysarthria), difficulty swallowing (dysphagia), drooping eyelids (ptosis), and double vision (diplopia). Patients often have nasal-sounding speech and weak neck muscles that give the head a tendency to fall forward or backward. Weakness can make eating unpleasant and tiring and may cause choking. Muscles that control facial expressions may be affected making it difficult to smile. These symptoms which occur in most people with myasthenia gravis tend come and go and may disappear for weeks and then recur.
Generalized weakness often develops in the trunk, arms, and legs within a year of onset. Arm muscles usually are affected most severely. It may become difficult to lift the arms over the head, rise from a sitting position, walk long distances, climb stairs or grip heavy objects. Muscle weakness tends to worsen as the day progresses, especially after prolonged activity. Symptoms fluctuate and are typically worse in hot weather, during or immediately after an infection, or during menstruation.
Myasthenia gravis itself does not cause pain, but the weakness may lead to non-specific aches and pains. For instance, neck pain may occur because of weakness in the neck muscles.
Only the voluntary muscles are affected by myasthenia gravis so the heart and the gastrointestinal tract are spared.
The symptoms of myasthenia gravis usually progress, reaching maximum or near-maximum severity within one to three years of onset in most people. Weakness serious enough to require full-time wheelchair use is not common and when properly treated, people with myasthenia gravis usually find they can remain physically active.
Remission occurs in about one fifth of people with myasthenia gravis. Usually, the remissions are temporary, with an average duration of five years, but some people experience more than one remission during their lifetime. A few people have experienced apparently permanent remissions, lasting more than 20 years.
What complications can occur?
Myasthenic crisis is a medical emergency that develops when muscles that control breathing become severely weakened. This may lead to acute respiratory failure and patients often require a respirator to assist breathing during the crisis. Other complications that may develop include choking, food aspiration, and pneumonia.
Factors that can trigger complications in patients with myasthenia gravis include illness (e.g., viral respiratory infection), surgery, corticosteroid use that is tapered too quickly, overexertion (especially in hot weather), pregnancy, and emotional stress.
When myasthenia is properly treated, crisis is very rare. And when crisis does occur, it has a good rate of recovery thanks to the wide range of treatments and the quality of respiratory care at most hospitals.
Is it risky for women with myasthenia gravis to have children?
Myasthenia gravis symptoms sometimes get worse during pregnancy but equally often get better or stay the same. Some medications for myasthenia gravis are safe to use during pregnancy and breastfeeding, but some others aren’t recommended, so this should be discussed with your doctor.
Between 10 and 20 percent of babies born to mothers with myasthenia gravis are born with a temporary form of myasthenia gravis, due to the transfer of antibodies across the placenta. These symptoms respond well to treatment and usually disappear within days to weeks causing no permanent disability.
Who gets myasthenia gravis?
Myasthenia gravis affects all races and can develop at any age from childhood to old age. Women are affected nearly three times more often than men during early adulthood (under 40 years of age). After 50 years of age, more men are affected than women. It is fairly unusual for children under the age of 15 to have myasthenia gravis; except in some Asian countries where up to half of patients have symptoms beginning in childhood.
Although the condition doesn’t generally run in families, people who inherit a tendency to develop autoimmune conditions are at increased risk of developing myasthenia gravis, so a patient with myasthenia gravis may have another autoimmune disease, such as diabetes, or have a relative with autoimmune disease.
A 2009 survey found that 2574 people in Australia were currently being treated for myasthenia gravis. This corresponds to approximately 1.2 out of every 10, 000 people.
What causes myasthenia gravis?
Myasthenia gravis is just one of many autoimmune diseases, which include arthritis and type 1 diabetes. Normally, the immune system produces antibodies that recognise foreign things that enter the body, such as bacteria and viruses. This leads to them being destroyed and cleared from the body. In the case of an autoimmune condition, the body’s immune system produces antibodies against things in the body that aren’t foreign. In myasthenia gravis it is the structure at the junction of the nerves and the muscles (the neuromuscular junction) that is attacked.
About 85 percent of patients with myasthenia gravis produce antibodies against a protein called the ‘acetylcholine receptor’ (AChR). This is found at the neuromuscular junction and acts as a receiver for the chemical signal ‘acetylcholine’ (ACh) that is released from the nerve to tell a muscle to contract. The antibodies bind to the acetylcholine receptors on the surface of the muscle and greatly reduce their ability to receive the chemical signal. As a result the patient experiences muscle weakness which becomes worse as they repeatedly try to use the same muscle.
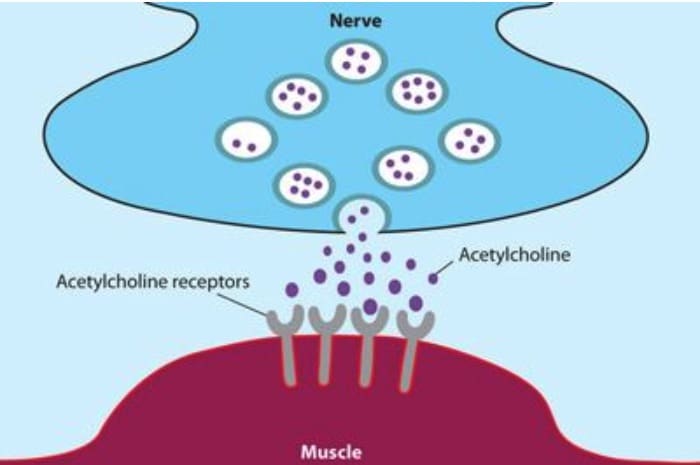
The neuromuscular junction
Many people with myasthenia gravis who don’t have antibodies to the AChR have antibodies to a protein called ‘muscle-specific kinase’ (MuSK). This protein helps organise ACh receptors on the muscle cell surface. Research is ongoing to find out what antibody is responsible in the approximately 10 percent of patients who don’t have antibodies to AChR or MuSK. Recently antibodies to a protein called ‘LRP4’ were found to be the cause for some of these patients.
Scientists don’t know what triggers most autoimmune conditions, but they have a few theories. One possibility is that certain viral or bacterial proteins mimic ‘self-proteins’ in the body (such as AChR), stimulating the immune system to accidentally attack it.
There is also evidence that the thymus gland plays a role in myasthenia gravis. About 15 percent of people with myasthenia gravis have a thymic tumour, called a thymoma, and another 65 percent have an overactive thymus, a condition called thymic hyperplasia. When the thymus doesn’t work properly, the immune system might lose some of its ability to distinguish self from non-self, making it more likely to attack the body’s own cells.
How is myasthenia gravis treated?
Myasthenia gravis is one of the most treatable neuromuscular disorders. The choice of treatment depends on several factors, including age, overall health, severity of disease, and rate of disease progression.
Anticholinesterase medications
The most commonly prescribed anticholinesterase medication is pyridostigmine (Mestinon). These drugs prevent ACh destruction and increase the accumulation of ACh at neuromuscular junctions, improving the ability of the muscles to contract. The benefits of Pyridostigmine occur within 30 to 60 minutes, but wear off in 3 to 4 hours, so tablets should be taken at regular intervals throughout the day
Side effects include excessive salivation, involuntary muscle twitching (fasciculation), abdominal pain, nausea, and diarrhoea. Other drugs may be used with anticholinesterase medications to reduce gastrointestinal side effects.
Immunosuppressants
Corticosteroids (e.g. prednisone) suppress the antibodies that interfere with the function of the neuromuscular junction and may be used in conjunction with anticholinesterase medication. Corticosteroids improve symptoms within a few weeks and once improvement stabilises, the dose is slowly decreased. A low dosage may be used indefinitely to treat myasthenia gravis; however, side effects such as gastric ulcers, osteoporosis (bone thinning), weight gain, high blood sugar, and increased risk of infection may develop over the long term.
Azathioprine (Imuran) is a non-steroidal immunosuppressant. It acts more slowly than corticosteroids, producing improvement after three to six months, and usually has few side effects. Occasionally, however, it can produce serious side effects such as inflammation of the pancreas, liver toxicity, bone marrow suppression and possibly an increased risk of cancer.
Mycophenolate mofetil (CellCept) is a relatively new immunosuppressant, but so far it has shown promising results against myasthenia gravis in clinical trials with the majority of patients experiencing gains in strength or a reduced need for prednisone after taking it for several months. Side effects occur occasionally and are relatively minor, such as stomach upset, flu-like symptoms, rash and tremor.
Cyclophosphamide and Cyclosporin are considered effective against myasthenia gravis but because of their side effects they are only prescribed as a last resort when other medications are not effective.
Plasmaphoresis and IVIg therapy
Plasmaphoresis or plasma exchange is a procedure in which antibodies are removed from the bloodstream. It can be used to treat severe worsening of symptoms (exacerbations) or in preparation for surgery. Typically, two to three litres of blood is removed and separated into cells and plasma (liquid). The plasma is discarded and replaced with fresh frozen plasma, a blood product called albumin and/or a plasma substitute along with the collected blood cells. Most patients undergo several sessions over the course of two weeks or more. Plasmaphoresis improves myasthenia gravis symptoms within days and improvement lasts six to eight weeks. Risks include low blood pressure, dizziness, blurred vision, and formation of blood clots (thrombosis).
IVIg therapy is an injection of nonspecific antibody (immunoglobulin) that temporarily stops the immune system’s production of its own antibodies.
These treatments bring about fast, but short-lived relief from myasthenia gravis, and are mostly used until other medications take effect, prior to surgery or for myasthenic crisis. However, some people receive regular plasmapheresis or IVIg as a supplement to immunosuppressant drugs.
Thymectomy
Surgical removal of the thymus gland (thymectomy) is recommended for some patients. The thymus is located in the upper part of the chest below the larynx and above the heart. Approximately 15 percent of myasthenia gravis patients have a tumour of the thymus (thymoma) and 60 to 80 percent have abnormal enlargement (hyperplasia) of the thymus. Thymectomy is usually performed on patients with a thymoma and patients under the age of 55 with generalised myasthenia gravis. Benefits of thymectomy develop gradually and most improvement occurs years after the procedure is performed, but it is believed to be the only treatment capable of producing long-term, drug-free remission.
What is the prognosis?
Symptoms of myasthenia gravis usually progress to maximum severity within three years. After three years, patients usually stabilise or improve. The prognosis of myasthenia gravis has improved significantly with the introduction of immunosuppressive medication with the majority of patients becoming symptom free if they are adequately treated. However most patients do have to remain on tablets for life as the symptoms generally return if they stop the medication.
Is there anything I can do to stop my symptoms getting worse?
Myasthenia gravis cannot be prevented, but avoiding the following triggers may help patients prevent exacerbations:
- Emotional stress
- Overexertion
- Exposure to extreme temperatures
- Fever
- Illness (e.g. respiratory infection, pneumonia, tooth abscess)
- Low levels of potassium in the blood (hypokalemia; caused by diuretics, frequent vomiting)
- Medications (e.g., muscle relaxants, anticonvulsants, certain antibiotics, some heart drugs, local anaesthetics, magnesium salts including milk of magnesia).
When taking a new prescription drug for the first time, it is important to consult your doctor about its possible effects on myasthenia gravis. Also, you might want to keep a MedicAlert bracelet or card handy to inform emergency medical personnel that you have myasthenia gravis and that certain drugs can be harmful to you.
What research is being done?
Although the currently available myasthenia gravis treatments can usually keep symptoms under control, at least to some extent, the medications often have unwanted side effects and for some people may be ineffective. So researchers are searching for ways to rebalance the immune system by selectively switching off the harmful immune response rather than suppressing the whole immune system. This could lead to the development of gentler, but more effective myasthenia gravis treatments.
A lot of research is being undertaken to understand why autoimmune conditions occur and how they might be treated. In particular, large amounts of money are being invested into investigating more common autoimmune conditions such as arthritis and diabetes type 1 which may result in increased understanding for all autoimmune conditions and therefore treatments.
A clinical trial started in 2013 to test a drug called ‘Belimumab’ for myasthenia gravis. This drug was originally developed for lupus (SLE). It inhibits B cells which are responsible for part of the normal immune response, and also for the over-aggressive immune response in autoimmune diseases.
Other researchers are looking at other aspects of the immune system and whether they could be manipulated to dampen down the autoimmune response in myasthenia gravis. Most of this research is still at the laboratory stage but one approach involving a molecule called GM-CSF will soon be tested in myasthenia gravis patients in a small pilot study. More information here.
Other research efforts are focussing on optimising the currently available treatments and finding the optimal combinations and doses. Current clinical trials for myasthenia gravis are listed on clinicaltrials.gov – the U.S. National Institutes of Health website which lists most of the clinical trials happening around the world.





