Australians will be able to use rapid antigen tests for COVID-19 at home from 1 November 2021, once the Therapeutic Goods Administration (TGA) – our national medical regulator, approves this testing measure.
The TGA has already approved 33 rapid antigen tests for use under supervision from a health professional, which are already being used by some businesses.
What is a rapid antigen test, and more importantly, what is the international evidence about its effectiveness?
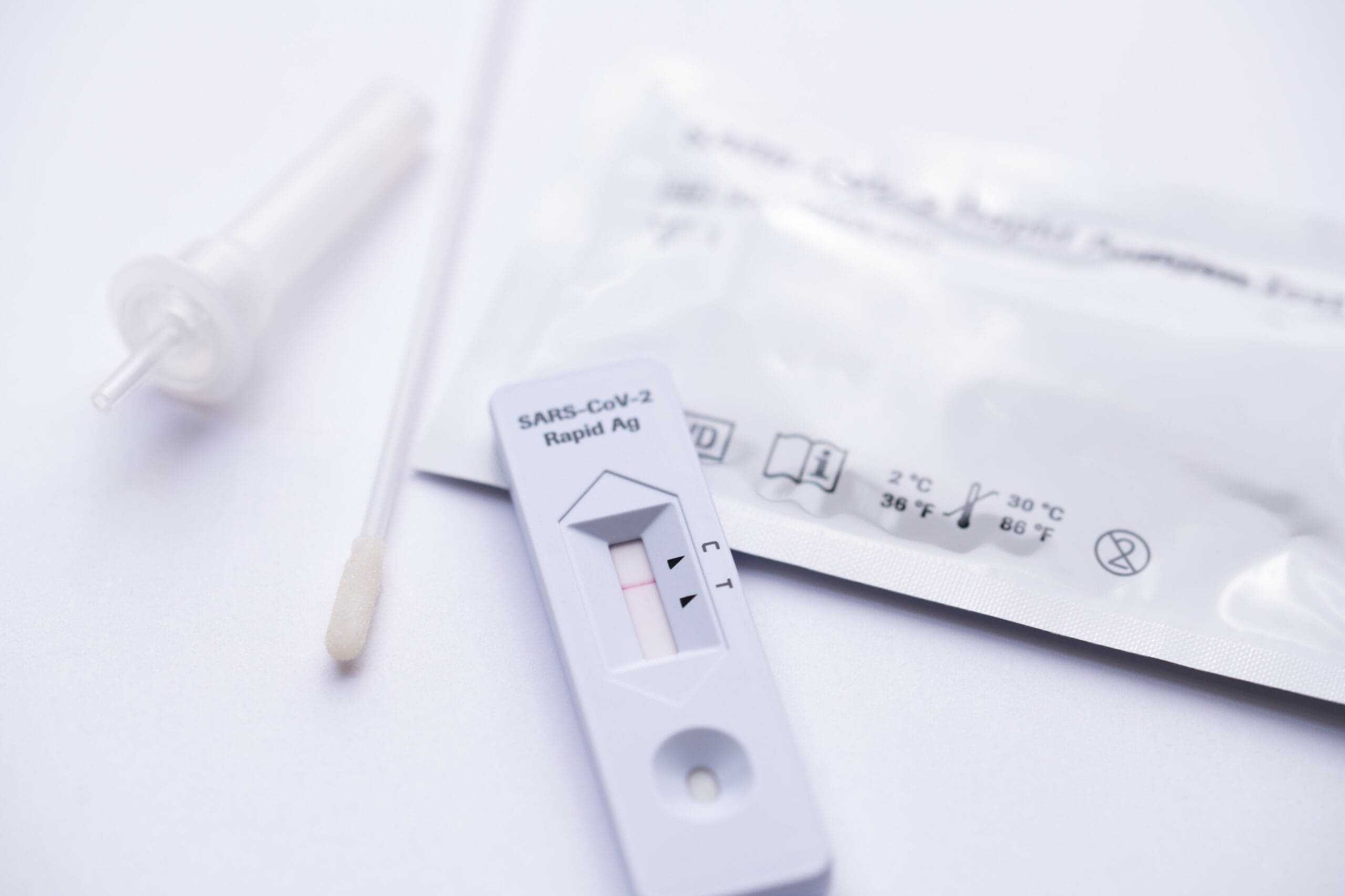
Rapid antigen tests are already used widely in Europe and the United States and can provide a result within 15 to 30 minutes.
Rapid antigen testing involves a nasal swab using a small cotton bud that is placed into a chemical solution. This is designed to detect a chunk of the virus itself. To get the result, you put that solution onto a reactive strip of paper or device, like a pregnancy test. The paper or device will then show you whether you are actively infectious with COVID-19, with about 80% accuracy – higher if you’re doing the test more regularly. There are also oral fluid tests that work in a similar way.
They cost between $10 – $15 compared to usually $85 for the polymerase chain reaction (PCR) tests.
The resistance to rapid antigen testing in Australia has mostly come from the pathology industry, which is concerned about the rapid tests not being accurate. However, the debate is changing overseas as evidence is showing that there is a place for such testing, especially for the workforce.
“What we found is that when using the rapid antigen tests 2-3 times a week the tests are as effective at finding people quickly in the course of their infection as any of the PCR tests. If done at a higher enough frequency the rapid tests can match the PCR tests for regular surveillance,” said Associate Professor Rebecca Smith, an Epidemiologist at the University of Illinois Urbana-Champaign.
Professor Iain Buchan, Executive Dean of the Institute of Population Health at the University of Liverpool also found the results of a study to be very encouraging.
“The rapid antigen tests were picking up about 8 in 10 likely infectious individuals which is pretty useful considering that test results would come back in 30 minutes, rather than 1 or 2 days later where a person could be spreading the virus in the community.”
Importantly, Professor Buchan’s pilot program found that the rapid tests saved 3200 staff days because people could go to work because they weren’t infectious, rather than working from home.
Health Minister Greg Hunt says rapid antigen tests would not replace PCR test but provide an additional option for people as restrictions are eased in many cities.
Further rules will be determined by state and territory governments, which will be able to take their own approaches.
Mr Hunt expects states to mandate that people who record a positive COVID-19 test result at home to undergo a PCR test for confirmation, and then isolate for a defined period of time.
New guidance for businesses considering rapid antigen testing has been released by the Therapeutic Goods Administration (TGA).