Australia has entered into several agreements for the supply of COVID-19 vaccines, if they are proved to be safe and effective. Learn more
Here is an update on the two vaccines that Australians will receive first.
Pfizer/BioNTech
The Therapeutic Goods Administration (TGA) has provisionally approved the Pfizer/BioNTech COVID-19 vaccine for use in Australia.
The approval is subject to strict conditions, and Pfizer will be required to continue providing information to the TGA on the safety, efficacy and quality of the vaccine.
The first priority groups include aged care and disability care residents and workers, frontline health care workers, and quarantine and border workers. They will start being immunised with the Pfizer vaccine in late February 2020 based on Australia’s COVID-19 vaccine national roll-out strategy
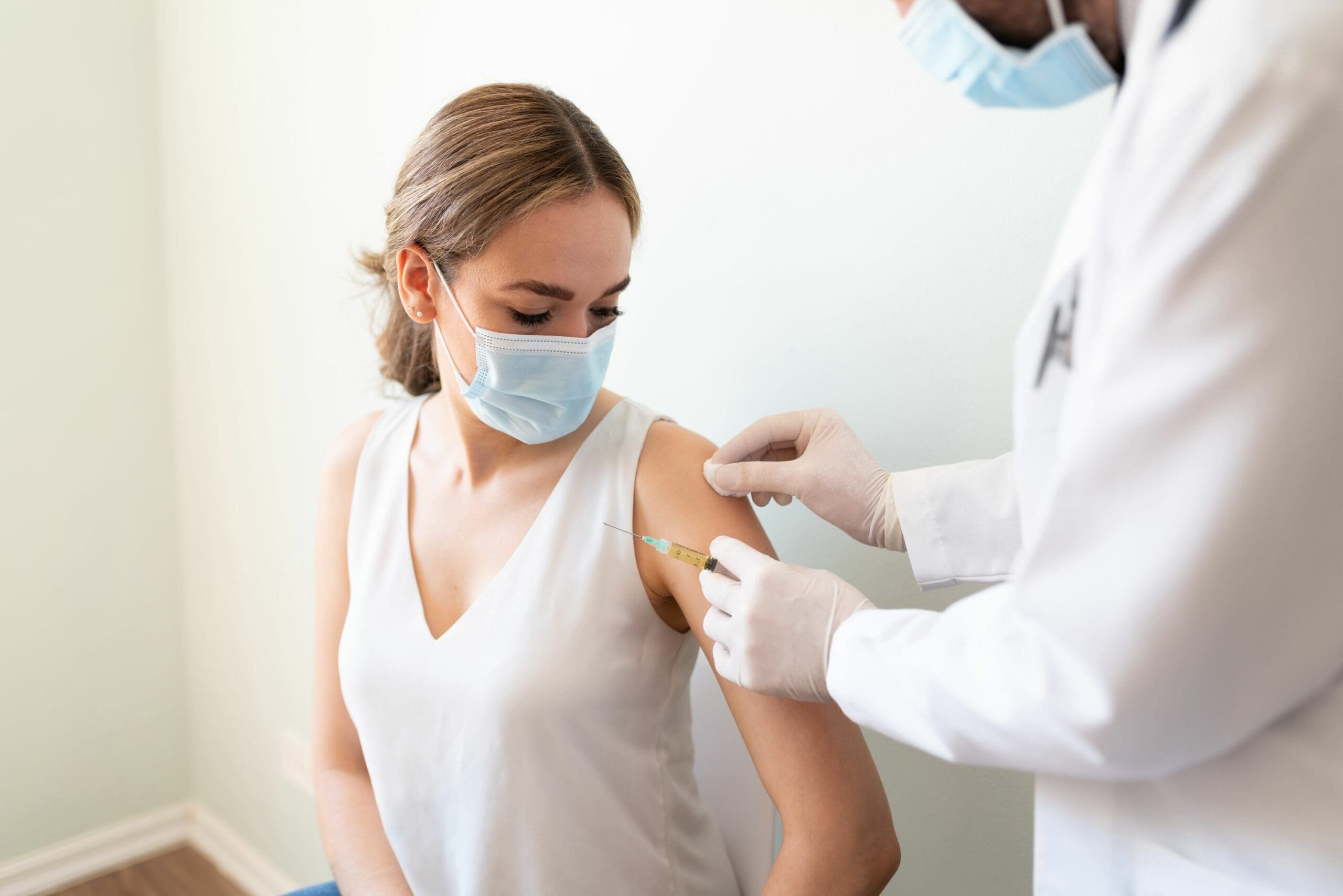
The Pfizer vaccine has been provisionally approved in Australia for people 16 years of age and over. Two doses will be required, administered at least 21 days apart.
About 20 million doses have been ordered, allowing for 10 million Australians to be vaccinated. 80,000 doses are planned for delivery this month. The schedule for the remaining doses is unclear at the time of writing.
The Pfizer vaccine will be delivered at up to 50 hospital sites across Australia, and in residential aged care and disability care facilities. Many general practices have expressed an interest in being vaccination hubs.
Oxford/AstraZeneca
The Therapeutic Goods Administration (TGA) is expected to provisionally approve the Oxford/AstraZeneca COVID-19 vaccine for use in Australia by March 2021.
The approval will also be subject to strict conditions, and Oxford/AstraZeneca will be required to continue providing information to the TGA on the safety, efficacy and quality of the vaccine.
Most Australians will receive the Oxford-AstraZeneca jab as part of Australia’s COVID-19 vaccine national roll-out strategy
Over 50 million doses have been ordered which will largely be locally manufactured by CSL in Melbourne and are expected to come on stream from late March 2021.
Two doses will be required, administered at least 21 days apart, although there is some evidence rthe vaccine works better with a longer space between doses (around 12 weeks).
The Oxford/AstraZeneca vaccine is expected to be available at GPs, medical facilities, pharmacies and health hubs.
What technology do the vaccines use?
Pfizer’s vaccine, like the Moderna vaccine uses a new technology involving messenger RNA (mRNA).
Instead of containing antigens like traditional vaccines, mRNA vaccines deliver the genetic material that codes for specific viral proteins, encapsulated in a lipid nanoparticle. In this case, the mRNA codes for the SARS-CoV-2 spike protein – the protein which enables the virus’ entry into host cells. The person’s cells are tricked into manufacturing the spike protein, which then triggers an immune response against the SARS-CoV-2 virus. The vaccine doesn’t touch the person’s own genes. It’s mimicking what the coronavirus does during an infection except that instead of the cell being told to produce the whole virus as in a natural infection, it just sends out the spike. These mRNA vaccines are very high performance and give a much better immune response than a natural infection.
These COVID-19 mRNA vaccines are the first vaccines to be approved using mRNA technology in humans.
The Oxford vaccine is called a viral vector vaccine. It also programs patients’ cells to produce the spike protein but instead of mRNA, it uses a chimpanzee common cold virus (an adenovirus) modified so that it cannot cause infection in humans which has been engineered to contain the genetic instructions for the spike protein.
How promising do the vaccines look?
Preliminary data from the companies show the Oxford vaccine is around 70% effective compared to 95% for Pfizer. However further studies and peer review are required. The comforting thing about all the vaccines so far is that they are highly effective at poreventing severe COVID-19 disease. Essentially they turn COVID-19 into the common cold for people who’ve been immunised. How well they prevent transmission of the virus is still an open question but it’s likely that they will but at a lower efficacy than prevention of disease.
Australian Government Department of Health. Australia’s vaccine agreements.
Australian Government Department of Health. Australia’s vaccine national rollout strategy
Pfizer [press release] Pfizer and BioNTech Achieve Approval by TGA for Their Vaccine Against COVID-19